SULFURREGEN™
The SulfurRegen™ process is a proprietary process which utilizes a sulfur recovery process. The SulfurRegen™ chemistry is a patented, wet scrubbing, liquid redox system that utilizes chemistry based around chelated iron. Through the use of this process, the H2S is converted into a sulfur slurry. This process does not use any toxic chemicals and does not produce any hazardous waste byproducts. The chemistry is continuously regenerated at a 30% more efficient rate than other liquid redox systems.The elemental sulfur can either be in liquid or "cake" form.
WHY SULFURREGEN™
The SulfurRegen™ process is ideal for producers that have consistent sulfur issues that require more capacity than a bubble tower, but not nearly enough to support an amine system. SulfurRegen™ takes advantage of lower Capex cost. This is either realized in upfront outlay or through our rental program. It also allows great flexibility in variable volume, as well as H2S levels, while not affecting economics. The SulfurRegen™ is applicable to all types of gas streams. The liquid catalyst adapts easily to change in flows and concentrations. SulfurRegen™ also has a proprietary automation system which controls contact time based on H2S levels, gas flow, and oxidation potential. Flexible operation allows for significant turndown in gas flow and concentration without loss of efficiency.
The APG-512 is an iron oxide, ferrihydrite nanoparticle (Fe3O3). Iron oxide may form in crystal formations such as goethite, lepidocrocite, maghemite, etc. These crystals follow pathways to nucleate and elongate. Ferrihydrite forms an amorphic nanoparticle with an undefined pattern, especially at the edges. Ferrihydrite cannot grow large (>1.0μm) and is in a quasi-solid state. This amorphic characteristic is critical to the reactivity of the molecules. This allows for increased efficiency without the tendency for crystal formation. Circulation, temperatures, and concentrations in the regenerator help to form ferrihydrite. Ferrihydrite is recognizable due to its burgundy color and its non-magnetic characteristic. Ferrihydrite remains suspended unlike other iron oxides that tend to precipitate rapidly.
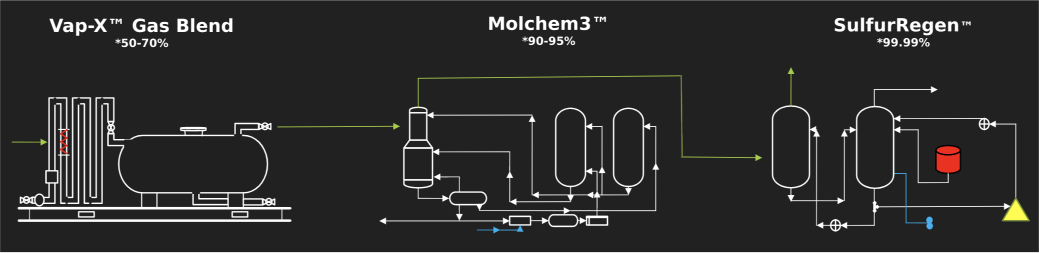
How It Works
1. H2S Capture From the Gas by APG-512
The overall reaction is summarized as a mole of ferrihydrite reacts with three-moles of H2S to form two-moles of iron-sulfide and one-mole of sulfur, thus each ferrihydrite results in the capture of three hydrogen sulfides.
2. APG-512 Solution Regeneration
The overall reaction is summarized as two-moles of iron sulfide react with one and a half moles of dissolved oxygen to form one-mole of ferrihydrite and two-moles of sulfur.
3. Sulfur Removal
The elemental sulfur that is precipitates through the process primarily falls to the bottom of the regenerator where it is gathered into the cone. The resulting solid formation is an iron/sulfur mixture that is non-hazardous and ready for disposal at local landfill with no special permits required.
APOLLO PETRO VS TRADITIONAL SOLUTIONS
| Benefits | SulfurRegen | MolChem3 | Triazine | Amine |
|---|---|---|---|---|
| Low Operating Cost | ✓ | ✓ | ✘ | ✓ |
| Low Energy Consumption | ✓ | ✓ | ✓ | ✘ |
| Low Chem Consumption | ✓ | ✓ | ✘ | ✓ |
| <2,000 lbs/S day | ✓ | ✘ | ✓ | ✘ |
| >2,000 lbs/S day | ✘ | ✓ | ✘ | ✓ |
| No Fall Out | ✘ | ✘ | ✘ | ✘ |
| Permanent Reaction | ✘ | ✘ | ✘ | ✘ |
| Fast Application Delivery | ✓ | ✓ | ✓ | ✘ |
| Non-Hazardous Reagent | ✓ | ✓ | ✘ | ✘ |
| Minimal Waste | ✓ | ✓ | ✘ | ✓ |
| Variable Volume Capability | ✓ | ✓ | ✘ | ✓ |
| Adapts to Process Variability | ✓ | ✓ | ✘ | ✓ |
| Easily Added to Existing Systems | ✓ | ✓ | ✓ | ✘ |
| Mitigates Scaling and Fouling | ✓ | ✓ | ✘ | ✘ |

